Tag Archive for: bioreference

OPKO Health Receives U.S. FDA Approval for the 4Kscore® Test
NewsMIAMI, Dec. 08, 2021 (GLOBE NEWSWIRE) -- OPKO Health, Inc. (NASDAQ: OPK) today announced that the U.S. Food and Drug Administration (FDA) has approved OPKO’s 4Kscore Test. This test is approved for use in men age 45 and older who have…

BioReference Laboratories Acquires U.S. Ariosa Centralized Laboratory Prenatal Testing Business
News
ELMWOOD PARK, N.J., August 16, 2021 – BioReference Laboratories, Inc., an OPKO Health company (NASDAQ:OPK), today announced the acquisition of the U.S. Ariosa centralized laboratory prenatal testing business from Roche.
Ariosa's…

Keeping Cancer Screening at the Forefront
BlogTo undergo cancer screening is a choice that we make- similar to diet, lifestyle, and other habits that can influence our overall health and well-being. However, the onset of the COVID-19 pandemic has resulted in disruptions to many aspects…
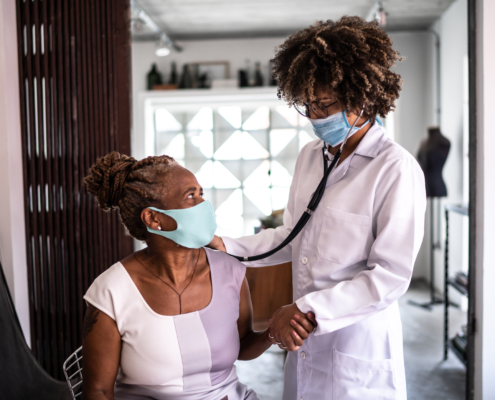
Acting FAST to Save from Strokes
BlogDid you know – Stroke kills twice as many women as breast cancer does, making stroke the third leading cause of death for women., according to the CDC.* May is American Stroke Awareness Month, a time to time to learn about the signs of stroke,…

Cervical Cancer Awareness Month
BlogJanuary is Cervical Cancer Awareness Month and a reminder that the most important thing you can do to prevent cervical cancer is to start screening at the age of 21. The American Cancer Society estimated that about 13,800 new cases of invasive…

OPKO Health’s BioReference Laboratories Launches Best-in-Class Next-Generation Sequencing (NGS) Assay
News, OncologyELMWOOD PARK, N.J., September 2, 2020 — BioReference Laboratories, Inc., an OPKO Health company (NASDAQ:OPK), along with its specialty oncology division, GenPath, today announced the launch of OnkoSight AdvancedTM, a next-generation sequencing…
