OnkoSight Advanced CompleteTM
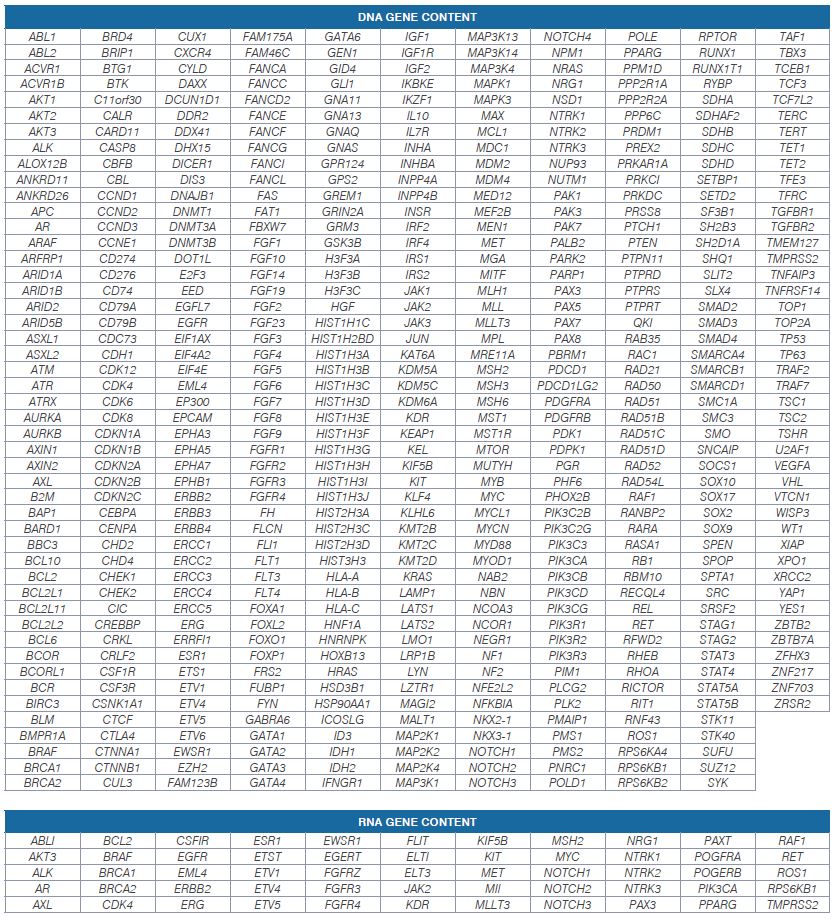
The OnkoSight Advanced CompleteTM is a 523-gene DNA and 55-gene RNA panel that is optimized to provide high sensitivity and specificity for low-frequency somatic mutation detection. DNA biomarkers include single nucleotide variants (SNVs), insertions, deletions, copy number variants (CNVs), and multinucleotide variants (MNVs).
OnkoSight Advanced Complete NGS Panel supports the identification of all relevant DNA and RNA variants implicated in various solid tumor types.
Key Advantages
| OnkoSight Advanced CompleteTM | Competition | |
| Guideline-recommended genes, including biomarkers for use with FDA-approved therapies | ✔ | ✔ |
| Customizable NGS Panel to meet healthcare providers’ and cancer patients’ needs | ✔ | X |
| Industry-leading turnaround time* | ✔ | X |
| Offered in conjunction with clinical and other pathology tests IHC, PCR, FISH, etc.) for a comprehensive cancer patient workup | ✔ | X |
*Clinical test results are available in 4-10 days. Reporting times are typical and begin once the specimen is received at the laboratory but could be extended in situations outside GenPath’s reasonable control.
Test Information
Test Code: TM57-2
Test performed in collaboration with PierianDx. Visit www.pieriandx.com to learn more.
GenPath® also offers single-gene testing and targeted NGS panel(s). Healthcare providers should only order panels if each gene or test in the panel is medically necessary.
Download a sample report here.

