GenPath® Oncology, now offers OnkoHRDTM homologous recombination deficiency (HRD) assessment with OnkoSight Advanced® next-generation sequencing (NGS).
- Measure genomic scars, which are defined by large-scale genomic instability, such as loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale state transitions (LST) in addition to the sequencing of the critical genes involved in the homologous recombination repair (HRR) pathway (e.g., BRCA1, BRCA2).
- Detect abnormalities across the genome, thereby expanding access to HRD status assessment of cancer samples.
- Interrogate CCNE1 Amplification status, a predictive biomarker for therapy resistance in epithelial ovarian cancer.
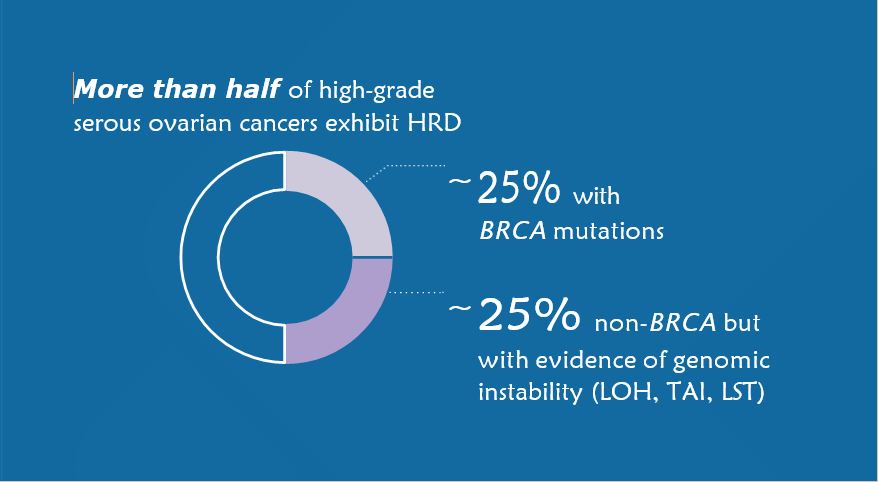
Per NCCN®, somatic testing in ovarian cancer should at a minimum include BRCA1/2, loss of heterozygosity (LOH), or HRD status in the absence of a germline BRCA mutation.
In ovarian cancers, HRD serves as a predictive biomarker for PARP inhibitors and a positive prognostic marker for both progression-free survival and overall survival.
FDA-Approved PARP Inhibitors Requiring HRD and/or HRR Gene Mutation Status:
Clients may add OnkoHRDTM (Test code TQ30-1) to any OnkoSight Advanced® panel listed in the table below. OnkoHRD must be ordered concurrently with OnkoSight Advanced® panel (Test code TP57, TH53, TK84, TH48, TJ16, or TM57) or within 30 days of sample receipt at the laboratory.
| TUMOR TYPE | THERAPY | GENPATH® TEST OPTION* |
|
Ovarian cancer |
Olaparib (Lynparza®)
Niraparib (Zejula®) Rucaparib (Rubraca®) |
TH53-1 OnkoSight Advanced® Gynecologic Neoplasms Panel
TJ16-5 OnkoSight Advanced® Solid Tumor Panel TM57-2 OnkoSight Advanced CompleteTM with PierianDx Interp |
|
Breast cancer |
Olaparib (Lynparza®)
Talazoparib (Talzenna®) |
TP57-6 OnkoSight Advanced® Breast Cancer Panel
TJ16-5 OnkoSight Advanced® Solid Tumor Panel TM57-2 OnkoSight Advanced CompleteTM with PierianDx Interp |
|
Prostate cancer |
Talazoparib (Talzenna®)
Rucaparib (Rubraca®) |
TH48-2 OnkoSight Advanced® Prostate Cancer Panel
TJ16-5 OnkoSight Advanced® Solid Tumor Panel TM57-2 OnkoSight Advanced CompleteTM with PierianDx Interp |
|
Pancreatic cancer |
Olaparib (Lynparza®) |
TK84-0 OnkoSight Advanced® Pancreaticobiliary Tumor Panel
TJ16-5 OnkoSight Advanced® Solid Tumor Panel TM57-2 OnkoSight Advanced CompleteTM with PierianDx Interp |
Please visit U.S. Food & Drugs website https://www.fda.gov/ to view full prescribing information of the abovementioned therapies.
*Includes somatic test options only. GenPath also offers OnkoRiskTM hereditary cancer testing. Please visit www.genpath.com to view GenPath’s comprehensive test offerings. Healthcare providers should only order panels if each gene or test in the panel is medically necessary and appropriate.
Test Information
OnkoHRD™ Homologous Recombination Deficiency Assessment
TQ30-1
NOTE: OnkoHRDTM can be ordered as an add-on test and must be ordered concurrently with TP57, TH53, TK84, TH48, TJ16 or TM57 or within 30 days of sample receipt at the laboratory.
OnkoSight Advanced® Gynecologic Neoplasms Panel | 50 genes, TMB, Tumor-only MSI, Virtual Karyotyping^
TH53-2
AKT1 |
AR |
ATM |
BRAF |
BRCA1 |
BRCA2 |
BRIP1 |
CCNE1 |
CDH1 |
CDK12 |
CHEK1 |
CHEK2 |
EGFR |
EPCAM |
ERBB2 |
ERBB3 |
ESR1 |
FANCA |
FANCC |
FANCL |
FGFR1 |
FGFR2 |
FOXA1 |
KRAS |
MLH1 |
MRE11A |
MSH2 |
MSH6 |
MUTYH |
NBN |
MYC |
NF1 |
PALB2 |
PIK3CA |
PMS2 |
POLE |
PTEN |
RAD50 |
RAD51B |
RAD51C |
RAD51D |
RAD54L |
RB1 |
RECQL4 |
SLX4 |
SMARCA4 |
TBX3 |
TERT |
TP53 |
XRCC2 |
OnkoSight Advanced® Breast Cancer Panel | 50 genes, TMB, Tumor-only MSI, Virtual Karyotyping^
TP57-6
AKT1 |
AR |
ATM |
ATR |
ATRX |
BARD1 |
BRAF |
BRCA1 |
BRCA2 |
BRIP1 |
CCNE1 |
CDH1 |
CDK12 |
CHEK1 |
CHEK2 |
EGFR |
EPCAM |
ERBB2 |
ERBB3 |
ERBB4 |
ESR1 |
FANCA |
FANCC |
FANCL |
FGFR1 |
FGFR2 |
FOXA1 |
KRAS |
MLH1 |
MRE11A |
MSH2 |
MSH6 |
MUTYH |
MYC |
NBN |
PALB2 |
PIK3CA |
PMS2 |
PTEN |
RAD50 |
RAD51 |
RAD51B |
RAD51C |
RAD51D |
RAD54L |
RB1 |
RECQL4 |
TERT |
TP53 |
XRCC2 |
OnkoSight Advanced® Prostate Cancer Panel | 49 genes, TMB, Tumor-only MSI, Virtual Karyotyping^
TH48-2
AKT1 |
AR |
ARID1A |
ATM |
ATR |
BARD1 |
BRAF |
BRCA1 |
BRCA2 |
BRIP1 |
CCND1 |
CCNE1 |
CDK12 |
CHD1 |
CHEK1 |
CHEK2 |
CTNNB1 |
EPCAM |
FANCA |
FANCL |
FANCD2 |
FAM175A |
FOXA1 |
FOXO1 |
GEN1 |
HOXB13 |
HRAS |
IDH1 |
MLH1 |
MRE11A |
MSH2 |
MSH6 |
MUTYH |
MYC |
NBN |
NTRK1 |
PALB2 |
PIK3CA |
PMS2 |
PPP2R2A |
PTEN |
RAD51B |
RAD51C |
RAD51D |
RAD54L |
RB1 |
SPOP |
TERT |
TP53 |
OnkoSight Advanced® Pancreaticobiliary Tract Panel | 23 genes, TMB, Tumor-only MSI, Virtual Karyotyping^
TK84-0
ARID1A |
BRAF |
BRCA1 |
BRCA2 |
CCNE1 |
ERBB2 |
FGFR1 |
FGFR2 |
FGFR3 |
IDH1 |
IDH2 |
KRAS |
MLH1 |
MSH2 |
MSH6 |
NRAS |
PALB2 |
PIK3C2G |
PMS2 |
STK11 |
TGFBR2 |
TP53 |
VHL |
OnkoSight Advanced® Solid Tumor Panel | 50 genes, TMB, Tumor-only MSI, Virtual Karyotyping^
TJ16-5
AKT1 |
APC |
ARID1A |
ATM |
BRAF |
BRCA1 |
BRCA2 |
CDH1 |
CDKN2A |
CTNNB1 |
EGFR |
EPCAM |
ERBB2 |
ERBB4 |
FBXW7 |
FGFR1 |
FGFR2 |
FGFR3 |
GNA11 |
GNAQ |
GNAS |
HRAS |
IDH1 |
IDH2 |
KDR |
KIT |
KRAS |
MEN1 |
MET |
MLH1 |
MSH2 |
MSH6 |
NOTCH1 |
NRAS |
PDGFRA |
PIK3CA |
PMS2 |
POLE |
PTEN |
PTPN11 |
RB1 |
RET |
SMAD4 |
SMO |
STK11 |
TERT |
TSC1 |
TSC2 |
TP53 |
VHL |
OnkoSight Advanced Complete® | 523-gene DNA, 55-gene RNA
TM57-2
Visit www.genpathdiagnostics.com/onkosight-advanced-complete/ to view the panel components
^Virtual karyotyping or CNA assessment will require samples with >50% tumor content. Normal findings will not be reported. For TJ16, CNA assessment will be performed on samples with an indication for tumor subtypes with HRD- associated therapy including high-grade serous ovarian (HGSO), fallopian tube, primary peritoneal, prostate, breast, and pancreaticobiliary carcinoma.
References:
- Stewart MD, Merino Vega D, Arend RC, Baden JF, Barbash O, Beaubier N, Collins G, French T, Ghahramani N, Hinson P, Jelinic P, Marton MJ, McGregor K, Parsons J, Ramamurthy L, Sausen M, Sokol ES, Stenzinger A, Stires H, Timms KM, Turco D, Wang I, Williams JA, Wong-Ho E, Allen Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist. 2022 Mar 11;27(3):167-174. doi: 10.1093/oncolo/oyab053. PMID: 35274707; PMCID: PMC8914493.
- Rempel E, Kluck K, Beck S, Ourailidis I, Kazdal D, Neumann O, Volckmar AL, Kirchner M, Goldschmid H, Pfarr N, Weichert W, Hübschmann D, Fröhling S, Sutter C, Schaaf CP, Schirmacher P, Endris V, Stenzinger A, Budczies Pan- cancer analysis of genomic scar patterns caused by homologous repair deficiency (HRD). NPJ Precis Oncol. 2022 Jun 9;6(1):36. doi: 10.1038/s41698-022-00276-6. PMID: 35681079; PMCID: PMC9184602.
- Gou R, Dong H, Lin B. Application and reflection of genomic scar assays in evaluating the efficacy of platinum salts and PARP inhibitors in cancer therapy. Life Sci. 2020 Nov 15;261:118434. doi: 10.1016/j.lfs.2020.118434. Epub 2020 Sep 14. PMID: 32941897.
- NCCN Clinical Practice Guidelines in Oncology Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer 2.2023- June 2, 2023. Accessed 7.26.2023
- Quesada S, Fabbro M, Solassol J. Toward More Comprehensive Homologous Recombination Deficiency Assays in Ovarian Cancer, Part 1: Technical Considerations. Cancers (Basel). 2022 Feb 23;14(5):1132. doi: 10.3390/ PMID: 35267439; PMCID: PMC8909526.


