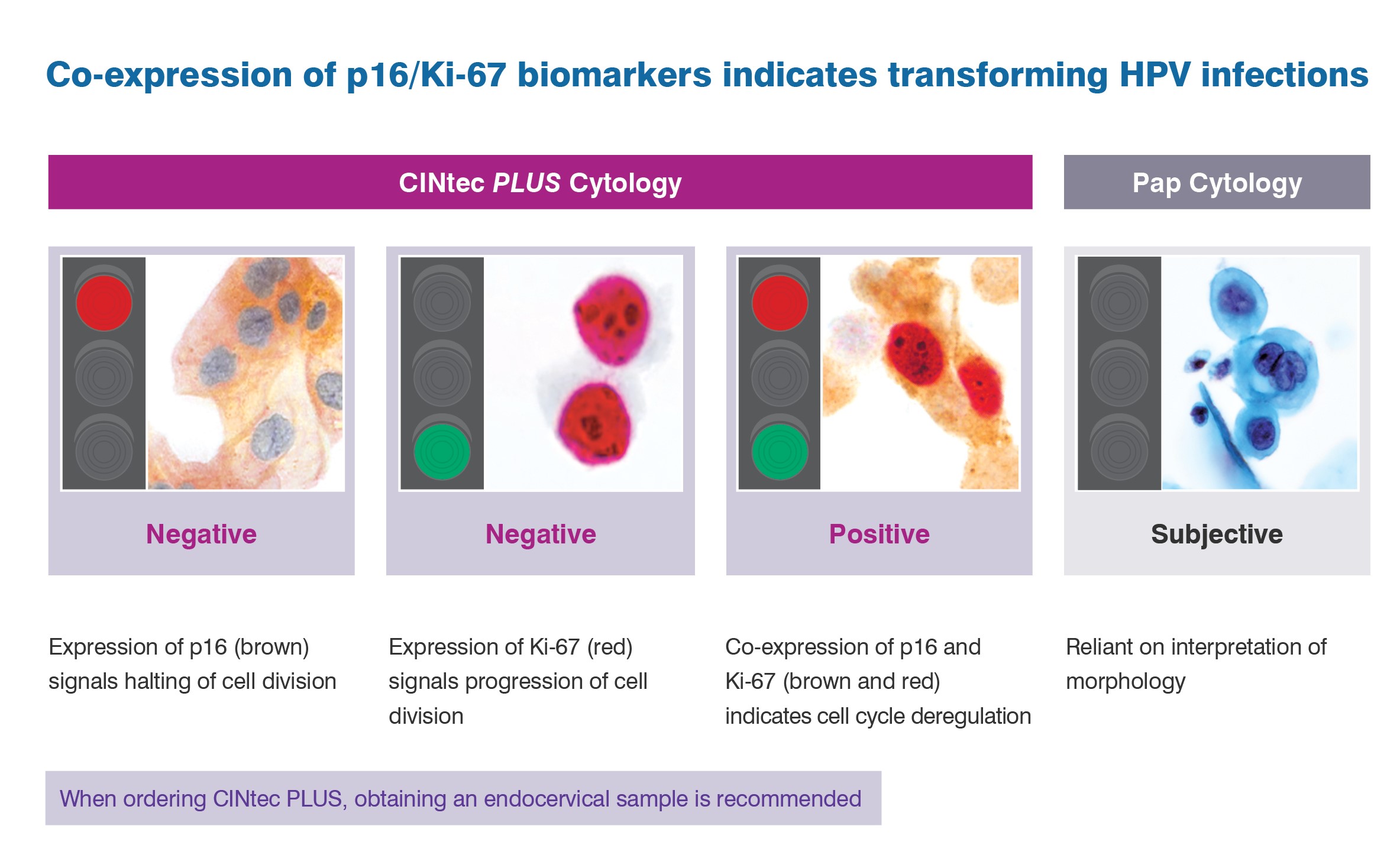
CINtec PLUS Cytology
A biomarker-based triage test to identify transforming HPV infection.
Not every HPV-positive patient will develop cervical cancer, so triage can determine who is most at risk and will benefit from more immediate follow-up, and who is at low risk and can be given more time to clear the infection on their own.
Detect changes at the cellular level:
The CINtec PLUS Cytology test is the only FDA approved triage test that uses dual biomarker technology to simultaneously detect p16 and Ki-67 in patients with HPV-positive results.
The co-expression of these two biomarkers within the same cell is a strong indicator that an HPV infection is undergoing oncogenic transformation.
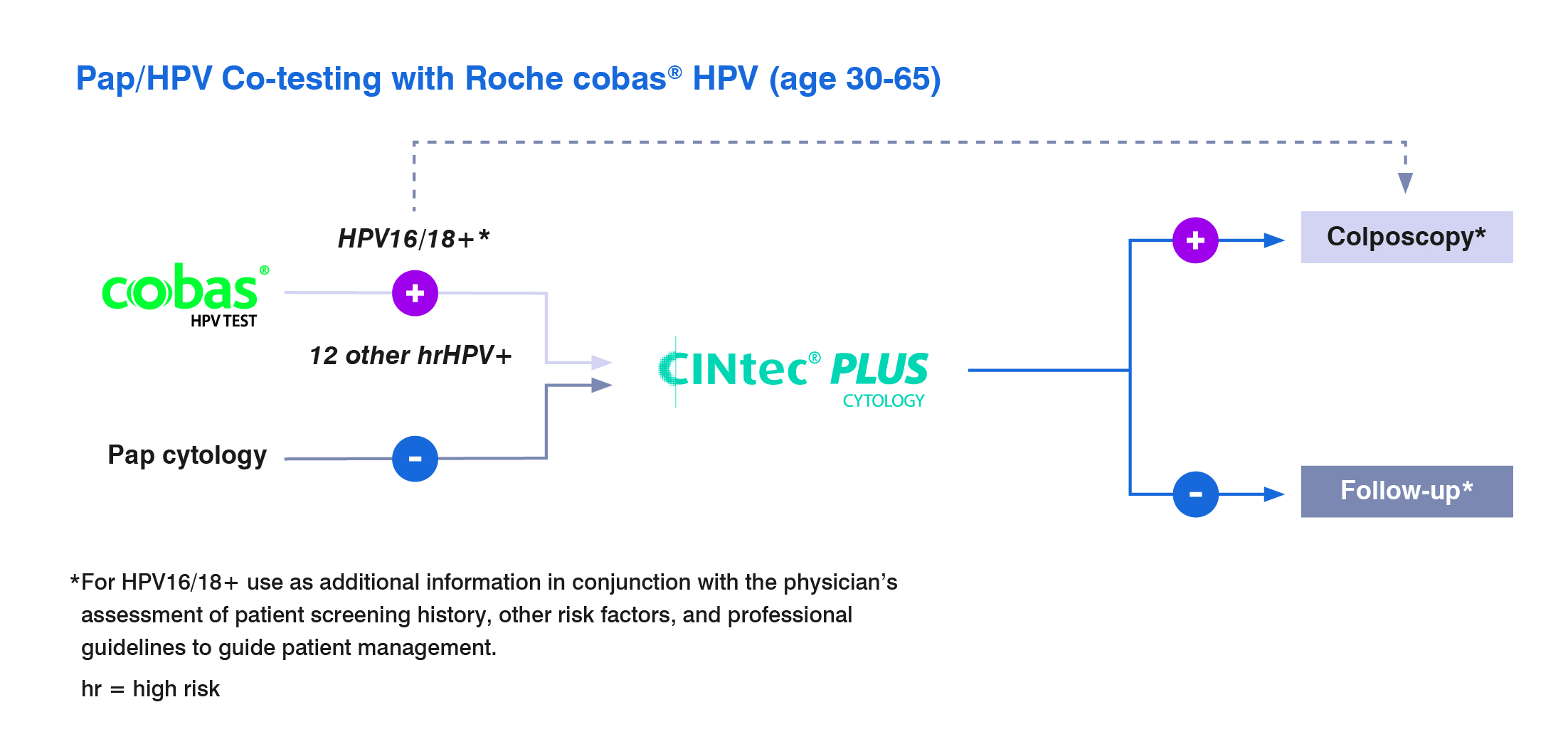
CINtec PLUS Cytology test is approved as a triage test with the cobas HPV test
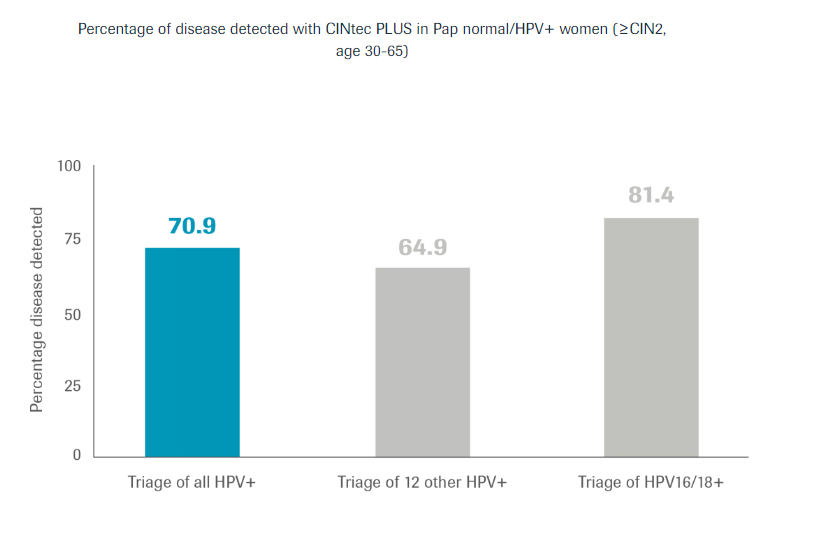
For discrepant Pap and HPV co-testing results of patients ages 30-65 when a patient is HPV positive but has a normal Pap.
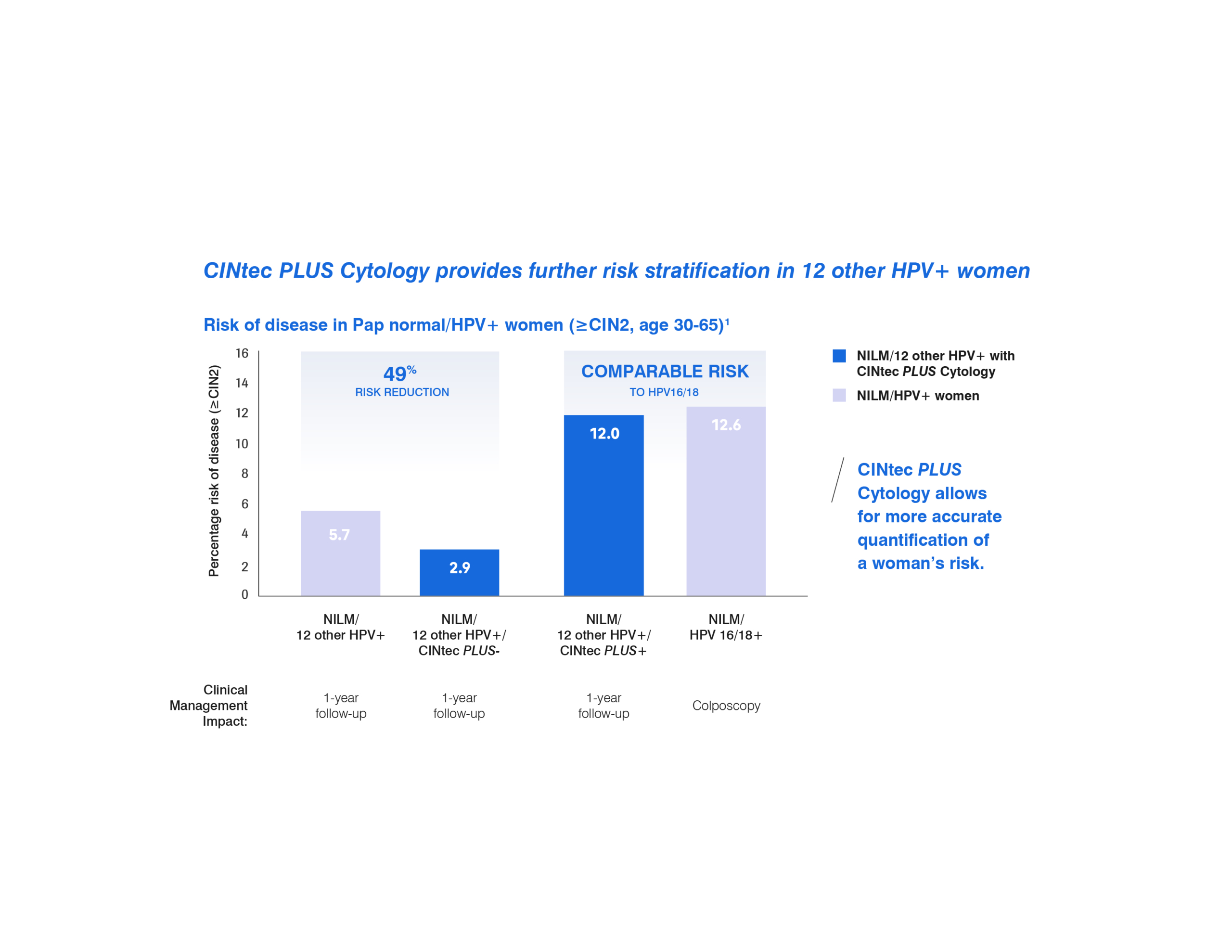
CINtec PLUS Cytology provides further risk stratification in 12 other HPV+ women.
The addition of the CINtec PLUS Cytology test can provide a more accurate quantification of a woman’s risk for high grade disease.
Increased testing sensitivity
When added as a triage to discrepant co-testing results, CINtec PLUS Cytology identified 7 out of 10 women as having cells undergoing oncogenic transformation allowing identification of disease earlier in their screening process.
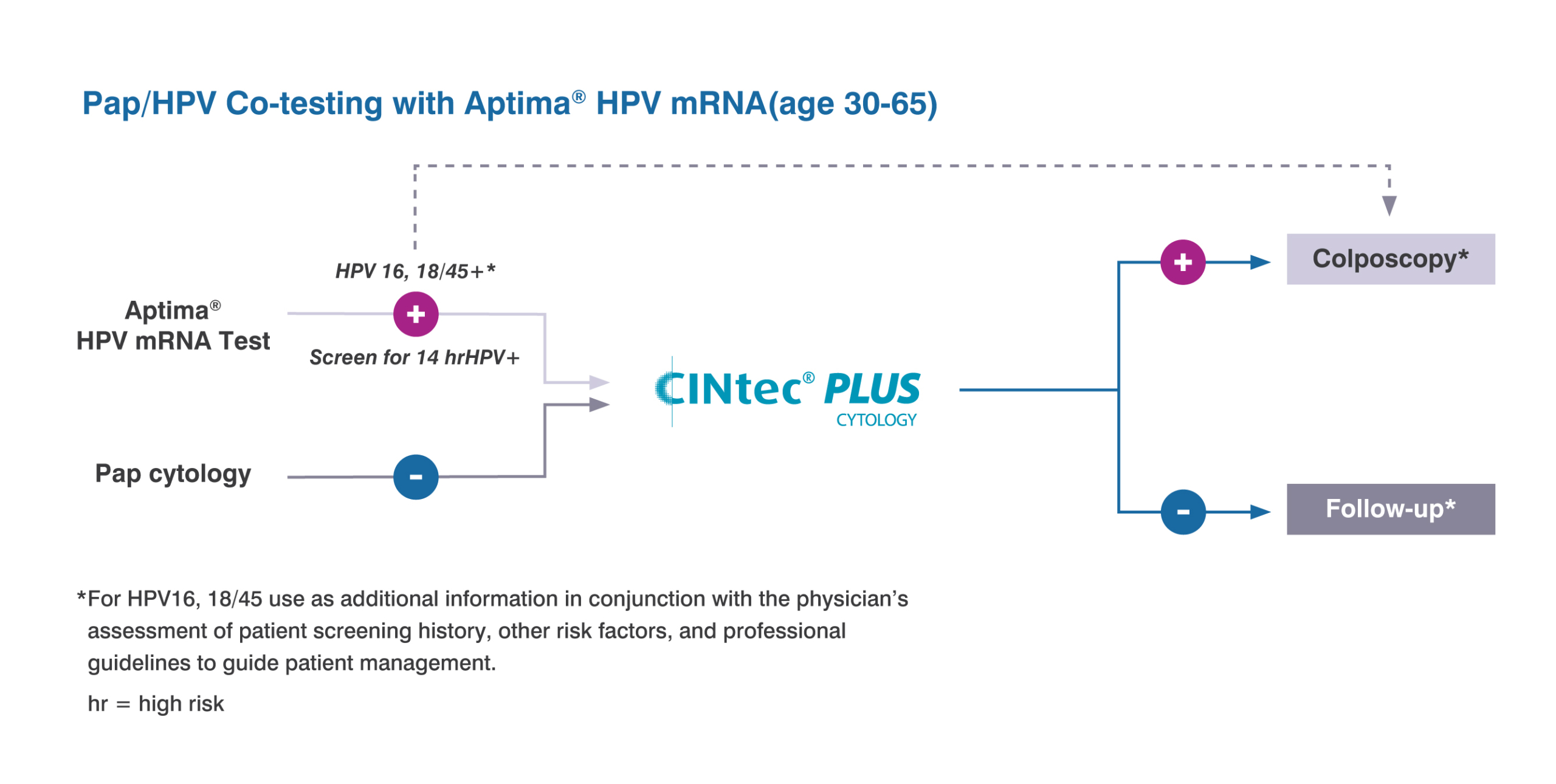
CINtec PLUS Cytology as a triage test with Aptima HPV of women ages 25-65 when HPV is positive.