OnkoSightTM Features
High Clinical Utility
Includes all gene mutations with clear therapeutic, diagnostic, or prognostic guidelines and those with established disease-associations for which there are clinical trial options
Cost-effective
Optimize efficiency and provide significant cost savings over competitive products
Low QNS Rate
OnkoSightTM can work with the most degraded of samples, ensuring that as many patients as possible who undergo a procedure for pathology testing have access to tumor profiling.
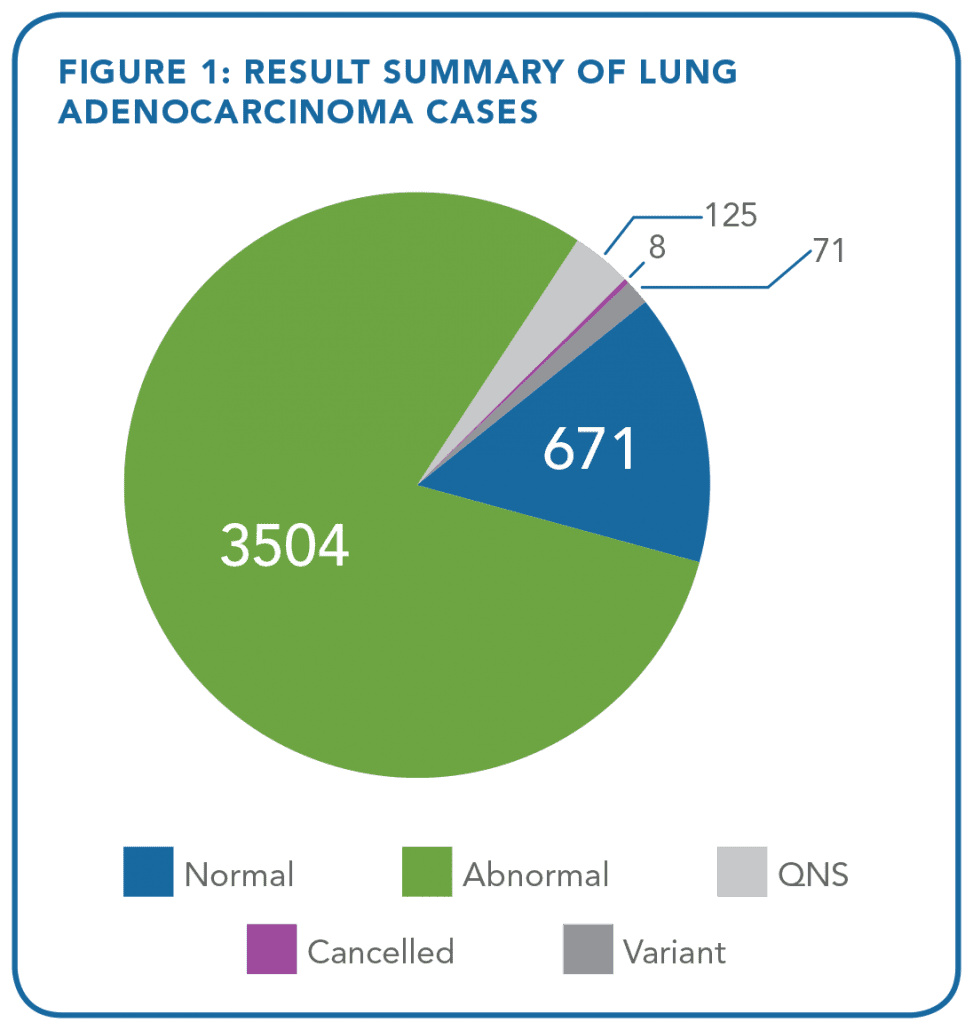
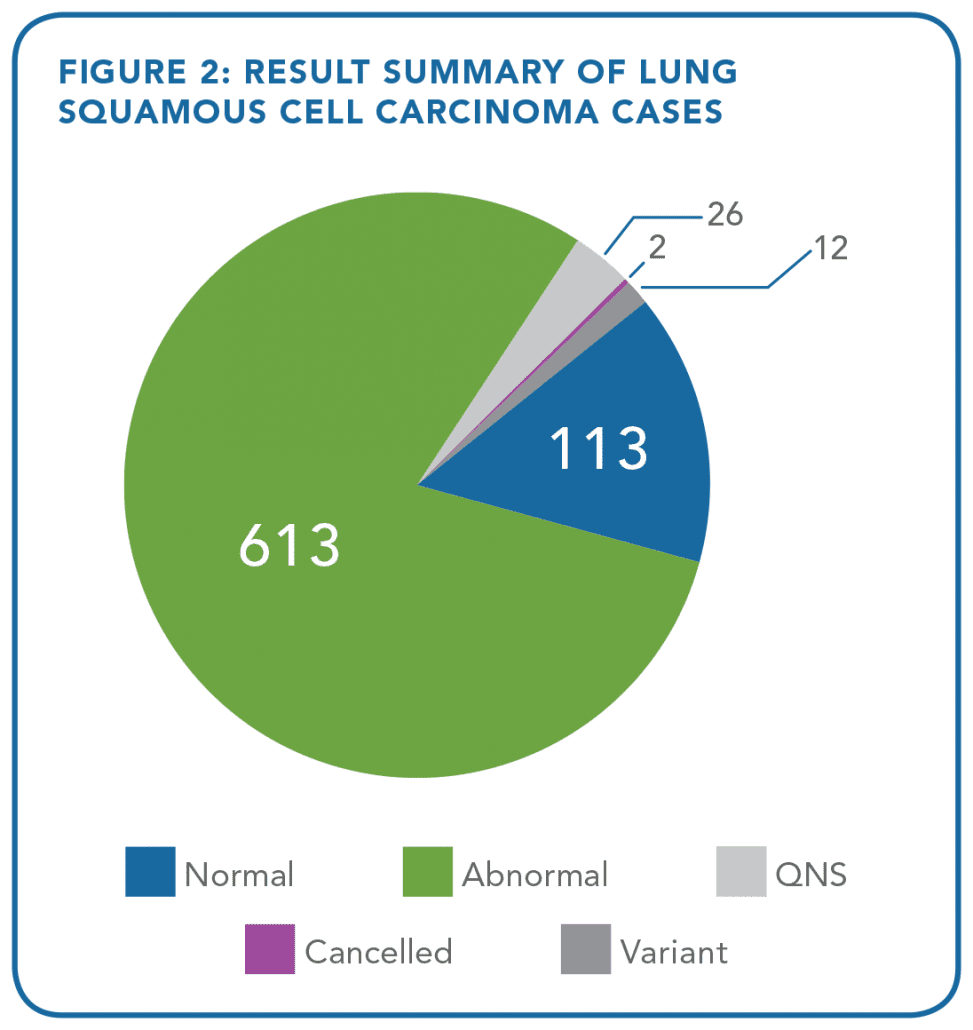
- In a retrospective analysis of 5,145 cases of non-small cell lung cancer (NSCLC) specimens (containing at least 10% tumor burden) profiled using the OnkoSightTM 18-gene lung cancer NGS panel from January 2015 to April 2018, 4,117 (80%) of the cases were found to have at least one somatic alteration. A total of 151 cases (2.9%) were deemed quantity/quality not sufficient (QNS) (Figures 1 and 2)
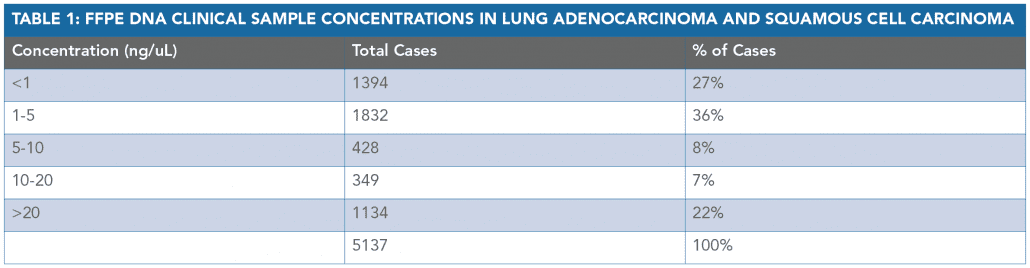
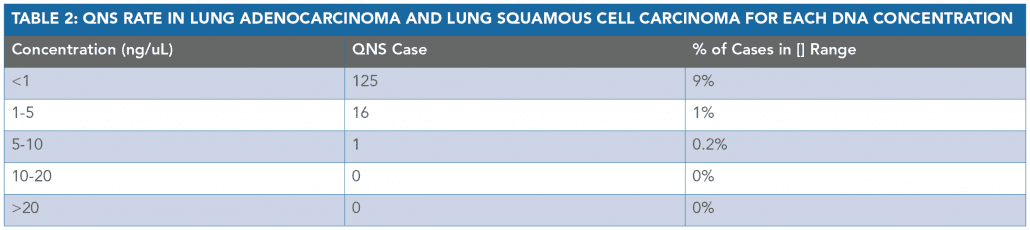
A result was obtained in 91% of lung adenocarcinoma and lung squamous cell carcinoma cases analyzed with only 1 ng/uL of DNA or less (Tables 1 and 2).
This data demonstrates an extremely low QNS rate and supports the clinical utility for OnkoSightTM NGS profiling in NSCLC, where FFPE samples are known often to be scant and depleted.
View the clinical paper that supports the above data below:
OnkoSight Features_AMP 2018 Poster
Accepted Specimen: In addition to Formalin Fixed Paraffin Embedded (FFPE) samples, Diff-Quick and Papanicolaou stained cytology smear slides are also accepted.
| Sensitivity & Specificity* | >99.9% at the listed limit of detection |
| Limit of Detection | 4% allelic burden for SNVs, insertions, and deletions |
| Depth of Coverage | Average: 2500x, Minimum at any loci: 250x |
| FFPE DNA Input | As low as ~1 ng (range of 0.5-20 ng) |
| Turnaround Time† | 4-6 days |
*If DNA meets laboratory quality control standards for degradation and quantification
†From receipt of acceptable specimen in the laboratory